Biomarker Testing Service
[vc_row][vc_column][vc_column_text]
Biomarker Testing Services
[/vc_column_text][vc_empty_space][/vc_column][/vc_row][vc_row][vc_column][vc_column_text]Order biomarker testing to determine effects of vaping as part of your e-cigarette or e-liquid ENDS PMTA application. Whether you seek biomarker testing services for the effects of vaping or smoking we offer ISO accredited biomarker testing services.
Biomarker Testing – Watch the video
Products in scope:
- ENDS (electronic nicotine systems)
- e-cigarettes
- nicotine containing e-liquids
- vaping devices
- cigarettes
Special features
– ISO accredited 17025 lab
– GLP and non-GLP testing
– e-cigarette/cigarette biomarker expertise
PMTA: Biomarker testing for effects of e-cigarettes
Under FDA rules, manufacturers are required to provide biomarker test data as part of their premarket tobacco application – or PMTA for their specific product.
What does the FDA guidance on PMTA say about biomarker testing?
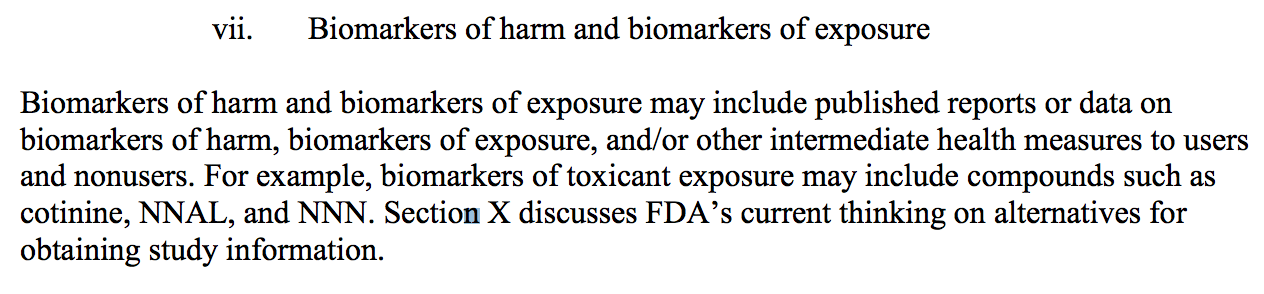
To evaluate the acute and chronic health effects associated with the product, FDA
recommends including studies, other scientific evidence, or both, that identify biomarkers of
exposure, biomarkers of harm, and health outcome measurements or endpoints. For example,
biomarkers of toxicant exposure may include compounds such as cotinine, NNAL, and NNN.
Source: PMTA guidance
Biomarkers of exposure and of potential harm
What are the biomarkers of exposure and biomarkers of potential harm? Analytes include:
- Nicotine, Cotinine and other nicotine metabolites
- 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL; a metabolite of nicotine-derived nitrosamine ketone NNK))
- 3-Hydroxypropylmercapturic acid (3-HPMA; a metabolite of acrolein)
- 11-dehydro Thromboxane B2 (11-dh-TXB2)
- 8-iso Prostaglandin F2α (8-epi PGF2α)
- and more…
[/vc_column_text][vc_empty_space][/vc_column][/vc_row][vc_row][vc_column][vc_empty_space][vc_column_text]
Extract from FDA guidance on biomarker testing
[/vc_column_text][vc_empty_space][/vc_column][/vc_row][vc_row][vc_column][vc_btn title=”Get a quote” style=”flat” shape=”square” color=”danger” align=”center” link=”url:https%3A%2F%2Fmedicpro.london%2Fcontact-us%2F|title:Contact%20Us||”][vc_empty_space][/vc_column][/vc_row][vc_row][vc_column][vc_column_text]Additional biomarkers of interest:
- PK analysis of nicotine in plasma
- N-Nitrosonornicotine (NNN)
- CEMA (Cyanoethylmercapturic acid, metabolite of acrylonitrile)
- AAMA/GAMA (mercapturic acid metabolites of acrylamide and glycidamide; metabolites of acrylamide)
- SPMA (Phenylmercapturic acid, metabolite of benzene)
- Aromatic amines
- OH-PAHs (metabolites of polycyclic aromatic hydrocarbons)
[/vc_column_text][vc_empty_space][/vc_column][/vc_row][vc_row][vc_column][vc_column_text]
Matrices of plasma, urine…
The following matrix options are available:
– plasma
– urine
– saliva
Test Method
LC-MS/MS – All methods are validated.
Turnaround time
20 – 25 working days (GLP testing may take longer)
Biomarker testing cost
The cost of biomarker testing depends on a number of factors including number of analytes, samples, frequency of testing and quality of the testing e.g. GLP or non-GLP. You can expect to pay in excess of £50,000 for data to support a PMTA application for a range of e-cigarettes or e-liquids.
About the Lab
- ISO 17025 Accredited Testing
- GLP and non-GLP testing available
- All methods are validated
[/vc_column_text][vc_empty_space][/vc_column][/vc_row][vc_row][vc_column][vc_btn title=”Get a quote” style=”flat” shape=”square” color=”danger” align=”center” link=”url:https%3A%2F%2Fmedicpro.london%2Fcontact-us%2F|title:Contact%20Us||”][vc_empty_space][/vc_column][/vc_row]
About Medic Pro
What We Do
We are a London-based regulatory affairs consultancy providing services to the e-cigarette, cosmetic, biocide, pharmaceutical and medical device industry. We help e-cigarette companies comply with the Tobacco Products Directive and pharmaceutical companies obtain and maintain medical product licences. We also offer UKAS accredited biocide and analytical testing services.