eCTD Publishing
eCTD Publishing Services
Whether you are looking to submit to FDA, EU or UK we can help you with eCTD publishing in line with the local regulations. Get in touch to arrange a consultation.
What is publishing in eCTD?
eCTD – or Electronic Common Technical Document – is the standard format for submitting applications, amendments, and reports to regulatory agencies like the FDA, EMA, and other Health Authorities. We offer eCTD publishing services to help applicants make eCTD submissions accurately and in a timely manner. Read on to learn more.
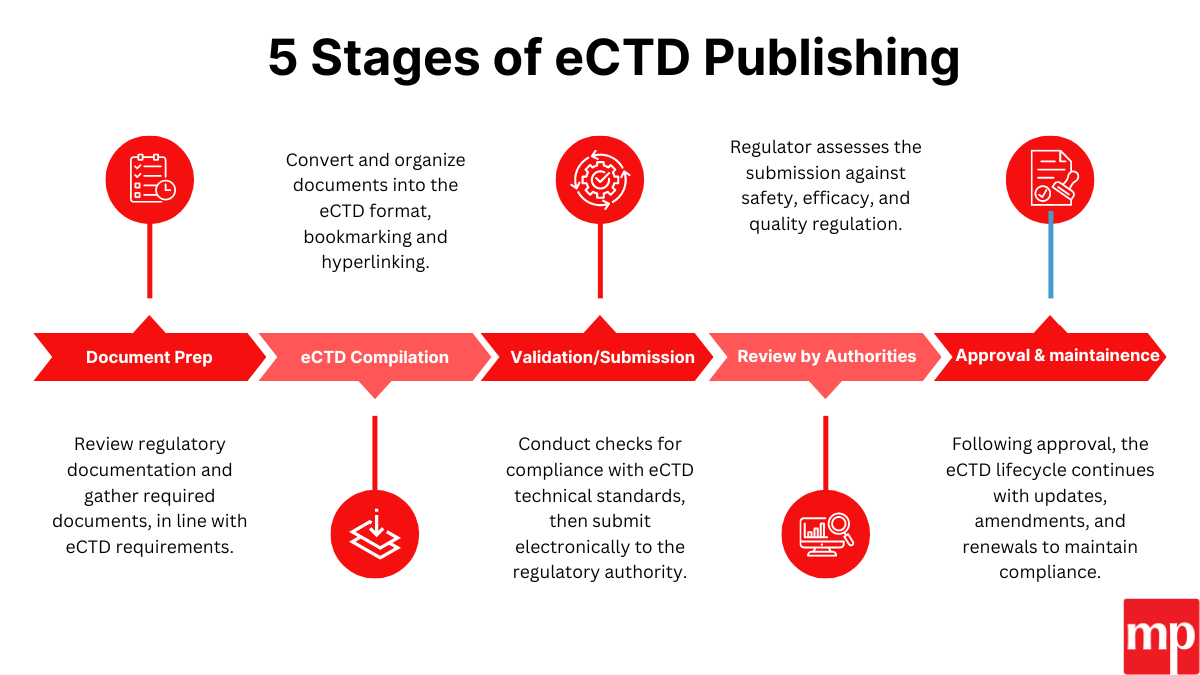
Our eCTD Publishing Services
We offer comprehensive services tailored to your needs:
- Multi-country eCTD filings
- Data compilation
- Compliance with both regional and ICH standards.
What we do
eCTD publishing requires a data gathering and compiling of product documentation for electronic submission. This process involves:
Project Management
We manage eCTD publication, from planning to submission, helping you to minimise delays.
Documentation Services
Our team specialises in formatting, editing, and preparing documents for eCTD submission, adhering to the specific requirements of the regulator.
Software Solutions
We use the latest eCTD software for reliable, efficient and compliant submissions.
Global Reach
With expertise in submissions in almost all territories where eCTD applies, that is Europe, USA, Canada, and some Arabian countries, we help to ensure global compliance for your submissions.
Contact us
Complete the form below or email info@medicpro.london and we’ll be in touch to discuss your eCTD publishing requirements.
Our eCTD Publishing Clients
From emerging biotech startups to established multinational corporations, over the years we have supported a wide variety of pharmaceutical companies with eCTD publishing. Our commitment to excellent customer support and efficiency has made us a trusted partner for companies seeking to navigate the complex regulatory submission process efficiently and effectively.
What is the eCTD?
Structured around an XML backbone, the eCTD streamlines the regulatory submission process by allowing for the integration of metatags, hyperlinks, and bookmarks. These features facilitate the review, and approval processes by the regulator.
Developed by the International Conference on Harmonisation – or ICH – the eCTD was introduced to replace inefficient paper submissions. The format harmonises the structure of pharmaceutical submission across multiple territories including US, EU and Japan.
What is XML in eCTD Publishing?
The technology behind eCTD publishing is the computer language called XML, which stands for Extensible Markup Language. XML is a flexible, structured language used to encode documents in a format that is both human-readable and machine-readable. Its adaptability makes it ideal for eCTD publishing.
XML allows for the efficient organisation and management of complex regulatory documentation. Its standardised structure allows for:
- Consistent presentation of data
- Interoperability
- Compliance with pre-determined regulations

eCTD Publishing: Which company should you choose?
We’ve prepare a checklist to help you identify the right eCTD publishing partner for you.
1) Flexibility and Scalability
Does the eCTD publishing vendor have the capability to pivot and adapt to your changing requirements? Can they take on new eCTD jobs without delay?
Our Approach: Our services are designed to adapt to your business needs, whether you’re a startup or a multinational corporation. We offer scalable solutions that grow with your business, ensuring that we can handle increasing volumes of submissions without compromising on quality.
2) Reliability and Trust
How dependable is the vendor? Will they deliver within the agreed timeframe consistently?
Our Approach: With years of experience in eCTD submissions, our team has earned the trust of businesses large and small. Our commitment to meeting deadlines and maintaining high-quality standards has made us a reliable partner in regulatory publishing.
3) “One-Stop-Shop” Service
Along side eCTD services, you may require further support to bring your product to market.
Our Approach: We provide a comprehensive service from eCTD to CMC advice and patient leaflet user testing.
4) Clarity and Comprehensiveness of Contract
Our Approach: Our contracts and work orders are designed to be transparent and straightforward, detailing the scope of services, timelines, and costs upfront. This clarity ensures that there are no surprises down the line.
eCTD Publishing Knowledge, Expertise and Credentials
Our Approach: Our team consists of industry experts with extensive experience and specialized credentials in regulatory affairs and eCTD publishing. This deep expertise ensures that we can navigate complex regulatory landscapes effectively.
Customer Support
Our Approach: We offer excellent customer services to support our clients from onboarding through to delivery and product lifecycle maintenance.
Ensure Support for Multiple eCTD Jurisdictions
Our Approach: Our eCTD services are designed to support submissions across a wide range of jurisdictions where eCTD submissions are mandatory.
FAQ
1. How do I submit an eCTD to the FDA, EU or UK?
- Prepare documents to meet ICH guidelines and format properly.
- Use specialist eCTD software compatible with agency specifications.
- Assemble submission, ensuring correct indexing and linking.
- Validate eCTD for errors and compliance with agency requirements.
- Submit through agency’s electronic portal or gateway.
- Await confirmation and feedback from regulatory agency.
2. What are the types of formats of regulatory submissions?
Regulatory submissions can be in the form of paper submissions, Non-eCTD electronic submissions (NEES), or Electronic submissions with eCTD (electronic common technical document).
3. Who developed the Common Technical Document (CTD)?
The CTD was developed by the European Medicines Agency (EMA), the Food and Drug Administration (FDA), and the Ministry of Health, Labour and Welfare in Japan.
4. What is the eCTD publishing format and its benefits?
The eCTD is an electronic transfer format for regulatory information, offering benefits like improved search functionality, tracking capabilities, reviewer efficiency, reduced approval time, and better handling of submissions.
5. What are the five modules of the CTD?
The five modules of the CTD are:
- Module 1: Administrative information (includes application forms, label mock-ups)
- Module 2: Overview and summaries (like a quick read version of other modules)
- Module 3: Quality (includes Chemistry, Manufacturing and Controls)
- Module 4: Preclinical (non-clinical) study reports (Toxicology)
- Module 5: Clinical efficacy (human studies).
6. Why is the recommended eCTD publishing format and what are its key advantages?
The eCTD format is highly recommended by the FDA due to its accountability, ease of navigation, paperless approach, space-saving benefits, transparency in submissions, and its acceptance as the standard format for new electronic submissions to CDER and DBER.
Resources
Contact us
Get in touch here or email info@medicpro and we’ll be in touch to discuss your eCTD publishing requirements.
You might also be interested in…
EU MDR (Medical Device Regulation)
Differences between UK MDR and EU MDR
Do you sell medical devices in the UK? Medical Devices manufacturers require a UK Representative to sell in the UK.

About Medic Pro
What We Do
We are a London-based regulatory affairs consultancy providing services to the e-cigarette, cosmetic, biocide, pharmaceutical and medical device industry. We help e-cigarette companies comply with the Tobacco Products Directive and pharmaceutical companies obtain and maintain medical product licences. We also offer UKAS accredited biocide and analytical testing services.

