Lilial Ban
Lilial ban in cosmetics: What you need to know
Lilial, also known as butylphenyl methylpropional (BMHCA), is a synthetic fragrance ingredient commonly used in cosmetics such as soaps, lotions, and shampoos.
Lilial ban in EU
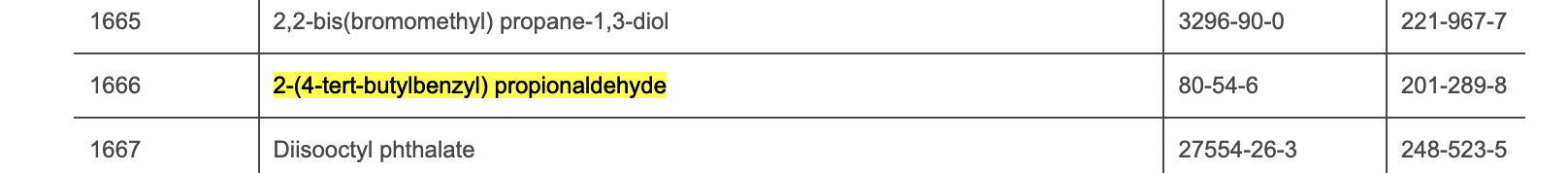
In 2021, the European Chemicals Agency (ECHA) added Lilial to its list of Substances of Very High Concern (SVHCs) due to its potential to be toxic to reproduction, cause skin sensitization and allergic reactions. Following this, the EU banned the use of Lilial in cosmetic products from 1 March 2022. EU cosmetic regulation has been updated to include Lilial (see below for entry).
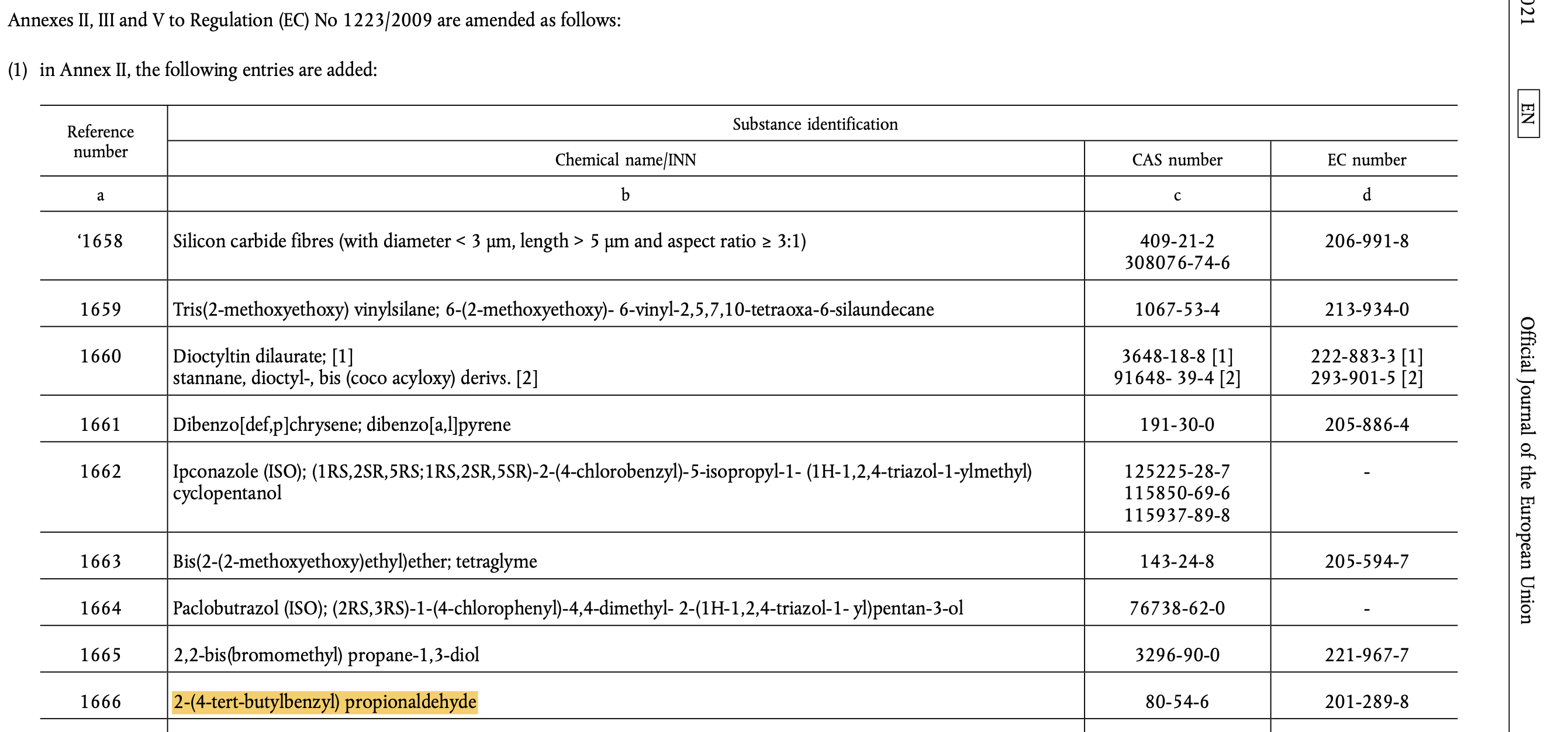
Note: 2-(4-tert-Butylbenzyl) propionaldehyde is the chemical name for Lilial.
SCCS opinion on Lilial
The body that reviews the safety of cosmetic ingredients in the EU, the SCCS, concluded back in 2015 that:
“BMHCA is not safe for use as fragrance ingredient in cosmetic leave-on and rinse-off type products, neither at concentration limits according to the ones set up by IFRA in 2013 (MoS = 3.6) nor at concentration limits as set up by IFRA in the revised proposal that has been submitted in 2015 belatedly (MoS = 53). In addition, no firm conclusion could be drawn on mutagenicity.“
Lilial ban in UK
Meanwhile in the UK, the Cosmetic, Toiletry and Perfumery Association (CTPA) advised its members to voluntarily remove Lilial from cosmetic products by the end of 2021. The UK’s cosmetic regulation has since been updated to include Lilial in Annex II LIST OF SUBSTANCES PROHIBITED IN COSMETIC PRODUCTS
What about other markets?
Lilial is still permitted for use in cosmetics in other countries outside the EU and the UK.
Want to learn more about cosmetic regulation and testing? Supercharge your knowledge with our online video guides.

Lilial ban: A timeline
2012: BMHCA evaluated under CoRAP
Where a manufacturer has concerns of the safety of a substance, the Community rolling action plan – or CoRAP – initiates an evaluation of the substance.
2015: The SCCS concluded “BMHCA is not safe for use as fragrance ingredient in cosmetic[s]”
The SCCS responsibility is to provide scientific opinion on health and safety risks of substances.
2019: Under CLP regulations, BMHCA is assigned the harmonised classification: reproductive toxicity, fertility, and Development (Repr. 1B).
Classification, Labelling and Packaging regulations – or CLP – communicates hazards to consumers.
2021: European Chemicals Agency (ECHA) identified Lilial as a Substances of Very High Concern (SVHCs) for its reprotoxic effects.
2021: EC No. 2021/1902 published which amendes Annex II of EU cosmetic regulation to include BMHCA in the Annex.
2022: UK added BMHCA to Annex II of the UK’s cosmetic regulation.
FAQ
Is lilial banned in the UK?
Yes, Lilial for use in cosmetics has been banned in the UK since the beginning of 2022. The chemical is included in the list of prohibited substances (Annex II) of the UK cosmetic regulation.
Is lilial banned in the EU?
Yes, the use of Lilial in cosmetics has been banned in the EU since March 2022.
Is lilial banned in the US?
No, Lilial is not currently banned in the US, but the FDA is monitoring the situation.
Is lilial banned in candles?
No, Lilial is not banned in candles.
Why is lilial banned?
Lilial has been banned due to its potential to be a reprotoxin, cause skin sensitization and allergic reactions.
What is lilial?
Lilial, also known as butylphenyl methylpropional (BMHCA), is a synthetic fragrance ingredient commonly used in cosmetics.
When was lilial banned?
Lilial was officially banned for use in cosmetic products in the EU & UK in 2022.
Is lilial dangerous?
Lilial has been linked to skin sensitization and allergic reactions in some individuals, which is why it has been banned in the EU and the UK.
Which olaplex products contain lilial?
It is unclear whether any Olaplex products contain Lilial, as the brand has not disclosed the full list of ingredients used in their products.
What is the date of the lilial ban?
The use of Lilial in cosmetic products has been banned in the EU since March 2022 and in the UK since the beginning of 2022.
What is the deadline for the lilial ban?
The deadline has now passed. No products containing lilial should be marketed in the EU and UK.
Other information
CAS No: 80-54-6 (containing two enantiomers, namely (2S)-3-(4-tert-butylphenyl)-2-methylpropanal (75166-30-2) and (2R)-3-(4-tert-butylphenyl)-2-methylpropanal (CAS 75166-31-
3)
EC No: 201-289-8
European Commission: Opinion on the safety of Lilial
Date of inclusion on SVHC list: 8th July 2021
Chemical name: 2-(4-tert-Butylbenzyl) propionaldehyde
INCI name: Butylphenyl methylpropional
IUPAC name: 3-(4-tert-Butylphenyl)-2-methylpropanal
Tradenames: Lilestralis, Lilial, Lysmeral®Extra
CLP: Danger! According to the harmonised classification and labelling (ATP15) approved by the European Union, this substance may damage fertility and is suspected of damaging the unborn child. Additionally, the classification provided by companies to ECHA in REACH registrations identifies that this substance is harmful if swallowed, is harmful to aquatic life with long lasting effects, causes skin irritation and may cause an allergic skin reaction.
CLP Hazard Class: Repr. 1B
What We Do
We are a London-based regulatory affairs consultancy providing services to the e-cigarette, cosmetic, biocide, pharmaceutical and medical device industry. We help e-cigarette companies comply with the Tobacco Products Directive and pharmaceutical companies obtain and maintain medical product licences. We also offer UKAS accredited biocide and analytical testing services.

