The SCCS recently finalised its evaluation of Benzyl Salicylate as an ingredient in cosmetics. Read on to learn background and implications of this regulatory update.
Benzyl Salicylate
Benzyl Salicylate (CAS No. 118-58-1, EC No. 204-262-9), also known as 2-hydroxybenzoic acid phenylmethyl ester, occurs naturally in various plants. It can be artificially manufactured and is often found in cosmetics, household items, and medicines. In cosmetics, it plays a vital role as a fragrance.
Past Evaluations and Regulatory Status
The SCCS last reviewed Benzyl Salicylate in 2012 and has been confirmed as an allergen in humans. Presently, Benzyl Salicylate is listed as an allergen in Annex III of the EU Cosmetics Regulation (entry 75). This means if its concentration exceeds 0.001% in leave-on products or 0.01% in rinse-off products, it must be explicitly listed in the ingredients on the label.
Are you new to cosmetic regulation and want to learn about regulations and testing? Check out our online video guides.

Call for Data
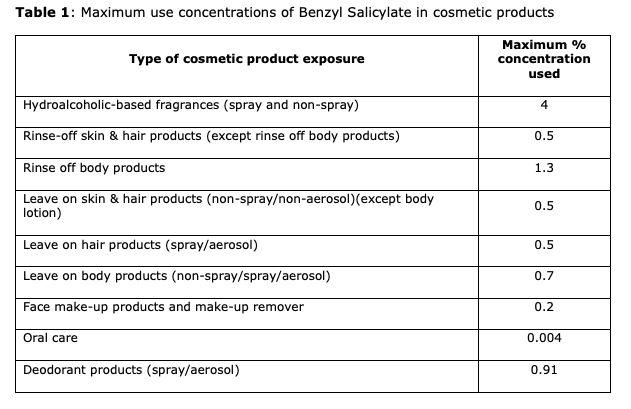
Recently, and as per the SCCS process, interested parties submitted toxicological evidence on the safety of Benzyl Salicylate as a fragrance ingredient in cosmetic products. In response, the Commission tasked the SCCS with conducting a safety assessment, considering the provided information. This assessment was to factor in the maximum concentrations of Benzyl Salicylate across different categories of cosmetic products, detailed in the table below.
SCCS Assessment
In consideration of the available data, the SCCS believes Benzyl Salicylate as safe up to the maximum concentrations outlined in the table above. Despite the presence of data suggesting an endocrine mode of action, no conclusive evidence indicates that this translates into observable endocrine effects.
Implications
Manufacturers must ensure cosmetics on the UK and EU market that contain Benzyl Salicylate adopt the findings of the SCCS and re-formulate in line with the recommendations. If reformulation is necessary, the safety cosmetic assessment must be amended by the cosmetic safety assessor and the PIF updated.
Contact us
Get in touch if you have questions regarding this update.
Resources
SCCS Opinion on Benzyl Salicylate
Entry in INCI database as ingredient and as substance
FAQ
1. What is Benzyl Salicylate, and where is it found?
Benzyl Salicylate (CAS No. 118-58-1, EC No. 204-262-9) is a compound naturally occurring in various plants and can also be artificially manufactured. It is commonly used in cosmetics, household items, and medicines, primarily for its fragrance properties.
2. What is the regulatory status of Benzyl Salicylate in cosmetics?
The Scientific Committee on Consumer Safety (SCCS) last reviewed Benzyl Salicylate in 2012, confirming it as an allergen in humans. Currently, it is listed as an allergen in Annex III of the EU Cosmetics Regulation (entry 75). If its concentration exceeds 0.001% in leave-on products or 0.01% in rinse-off products, it must be explicitly listed in the ingredients on the label.
3. What prompted the recent evaluation of Benzyl Salicylate by the SCCS?
Interested parties submitted toxicological evidence on the safety of Benzyl Salicylate as a fragrance ingredient in cosmetic products, prompting the European Commission to task the SCCS with conducting a safety assessment based on this new information.
4. What was the outcome of the SCCS assessment?
Based on the available data, the SCCS concluded that Benzyl Salicylate is safe up to maximum concentrations outlined in the assessment. While there were indications of a potential endocrine mode of action, no conclusive evidence suggests observable endocrine effects.
5. What are the implications for manufacturers of cosmetics containing Benzyl Salicylate?
Manufacturers must ensure their products comply with the SCCS findings. If reformulation is necessary to meet the recommended maximum concentrations, the safety assessment must be amended accordingly, and Product Information Files (PIF) must be updated.
6. Are there any specific concentration limits for Benzyl Salicylate in cosmetic products?
Yes, the concentration limits depend on the type of cosmetic product. For leave-on products, the limit is 0.001%, while for rinse-off products, it is 0.01%. Exceeding these limits requires explicit listing on the product label.



Comments are closed here.