Transpara AI Transforms Breast Cancer Diagnosis
Breast cancer is frequently misdiagnosed in women. According to the National Cancer Institute, about 20% of breast cancers are missed in mammograms (x-ray of the breast), leading to delayed treatment and higher risks of not curing the disease. However, with advancements in medical technology, software like Transpara can now detect cancer presence.
How does Transpara work?
Transpara, created by ScreenPoint Medical, is an AI software that helps with the detection of breast cancer. It uses an algorithm developed from millions of mammograms to determine whether cancer is present in a woman’s breast. The technology continuously learns from each mammogram it analyzes, identifying irregularities within minutes. Transpara uses a number scoring system to indicate the risk of breast cancer, with higher numbers on a 1-10 scale representing higher risk. Additionally, it offers computer-aided detection (CAD) to highlight suspicious abnormalities in breast tissue. Examples of Transpara’s detection capabilities can be seen in Figure 1.
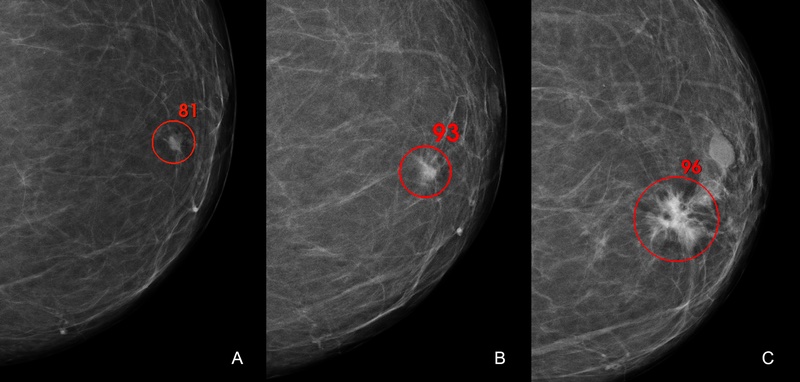
This image shows the use of Transpara technology to detect the presence of a lesion on a female breast and the possibility of cancer.
Trust and Clinical Validation
The Johns Hopkins Kimmel Cancer Center recently integrated Transpara software into their daily mammogram exams. Recognized as a “Center of Excellence” by the National Cancer Institute, the center saw improved screening workflow, decision confidence, and breast cancer diagnostic assessment after installing Transpara at seven locations. Transpara is now in use at over 50 sites, where research has shown that it helps detect 45% of cancers earlier.
FDA Clearance of Transpara
Transpara obtained their first FDA clearance in November of 2018 for use with 2D mammography and later received further clearance for 3D mammography in January 2020. It also has European MDR approval for use with both 2D and 3D mammography and is now utilized in over 30 countries to aid in breast cancer detection.
Clinician and Patient Benefits
AI technology like Transpara offers significant benefits for both patients and clinicians. Radiologists remain crucial in cancer detection, and Transpara serves as a supportive tool, saving valuable time and reducing workload. Patients benefit from the extra security in their mammogram interpretations, reducing misdiagnosis chances. Early treatment increases the likelihood of curing breast cancer.
AI technologies like Transpara are becoming essential in healthcare for both clinicians and patients. Transpara software reduces breast cancer misdiagnosis rates and allows healthcare professionals to allocate their time and efforts more effectively.
FAQ – Transpara AI Software
What is Transpara?
Transpara is an AI software by ScreenPoint Medical designed to detect breast cancer using advanced algorithms and computer-aided detection.
How does Transpara work?
Transpara analyzes mammograms with an algorithm developed from millions of images. It identifies irregularities and assigns a risk score from 1 to 10.
What are the benefits of Transpara for patients?
Patients benefit from reduced misdiagnosis rates, early cancer detection, and increased chances of curing breast cancer through timely treatment.
How does Transpara assist radiologists?
Transpara helps radiologists by improving decision confidence, saving time, and reducing their workload during mammogram analysis.
Where is Transpara in use?
Transpara is in use in over 30 countries, including installations at Johns Hopkins Kimmel Cancer Center and over 50 other sites.
What regulatory approvals does Transpara have?
Transpara received FDA clearance for 2D mammography in 2018, 3D mammography in 2020, and European MDR approval for both.



Comments are closed here.